41 when were food labels required
Food Labeling & Nutrition | FDA Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables) and... A Historical Look at Food Safety - IFT.org the federal food, drug, and cosmetics act of 1938 contained several new provisions, including: requiring safe tolerances be set for unavoidable poisonous substances, authorizing standards of identity, quality, and fill-of-container for foods, authorizing factory inspections, and adding the remedy of court injunctions to the previous penalties of …
How to Identify Gluten on Food Labels - Verywell Health The gluten-free food labeling requirements only apply to packaged foods. The rule doesn't apply to meat, poultry, unshelled eggs, or distilled spirits and wines made with 7% alcohol by volume or more. There is no standard symbol for gluten-free foods. Manufacturers can simply print "gluten-free" on their label as long as it is truthful.

When were food labels required
Organic on Food Labels - FDA Food products that are ordinarily under FDA's jurisdiction and labeled with organic claims must comply with both USDA NOP regulations for the organic claim and FDA regulations for food labeling and... CFR - Code of Federal Regulations Title 21 - Food and Drug ... The requirements in this section apply only to prescription drug products described in § 201.56(b)(1) and must be implemented according to the schedule specified in § 201.56(c), except for the requirement in paragraph (c)(18) of this section to reprint any FDA-approved patient labeling at the end of prescription drug labeling or accompany the ... What's New with the Nutrition Facts Label | FDA The U.S. Food and Drug Administration (FDA) has updated the Nutrition Facts label on packaged foods and drinks. FDA is requiring changes to the Nutrition Facts label based on updated scientific...
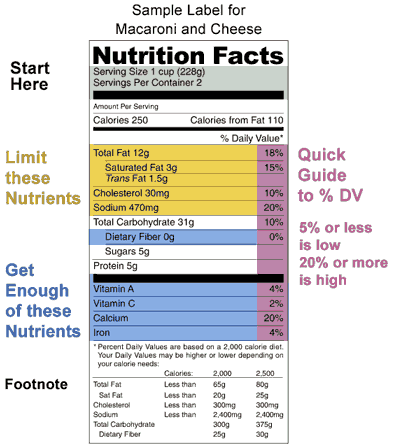
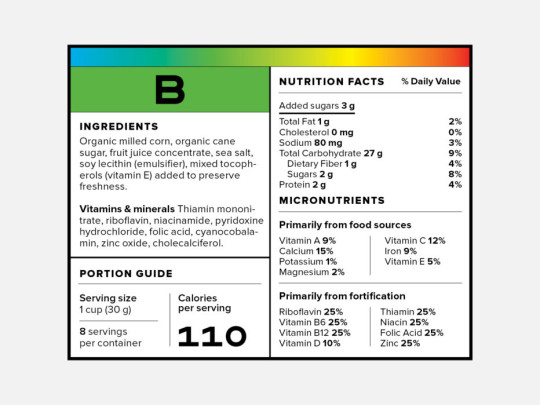
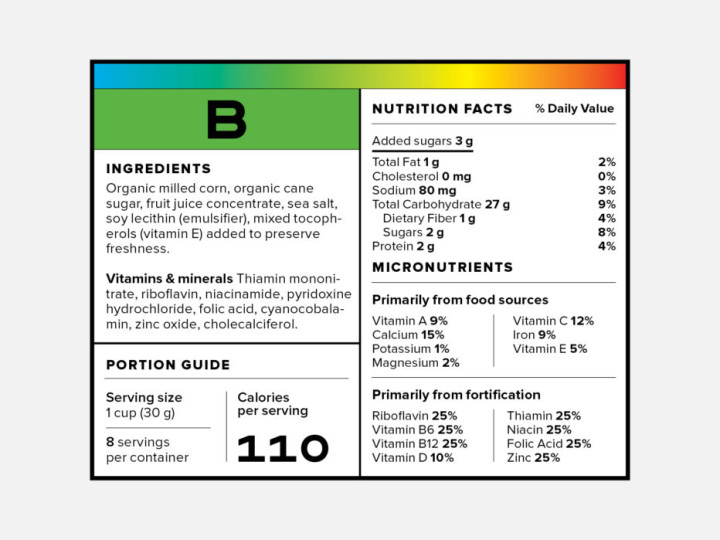
When were food labels required. China - Labeling/Marking Requirements Chinese regulators require imported and exported (but not domestic) food items such as candy, wine, nuts, canned food, and cheese to have labels verified and products tested for quality before goods can be imported or exported. Many products imported to China must receive a China Compulsory Certification (CCC) marking before the sale. The Basics of the Nutrition Facts Label The 2020-2025 Dietary Guidelines for Americans recommends that foods and beverages with added sugars be avoided by children under the age of 2 and individuals 2 years and older consume no more than 10% of daily calories from added sugars. Foods with more than one ingredient must have an ingredient list on the label. Federal Register :: Made in USA Labeling Rule This part shall not be construed as superseding, altering, or affecting the application of any other federal law or regulation relating to country-of-origin labeling requirements, including but not limited to the Federal Meat Inspection Act, 21 U.S.C. 601 et seq., the Poultry Products Inspection Act, 21 U.S.C. 451 et seq., and the Egg Products ... Understanding the USDA Organic Label | USDA Amidst nutrition facts, ingredient lists, and dietary claims on food packages, "organic" might appear as one more piece of information to decipher when shopping for products. Understanding what the organic label means can help shoppers make informed purchasing choices. Organic is a labeling term found on products that have been produced using cultural, biological, and mechanical practices ...
Dietary Guidelines for Americans | health.gov Food and nutrition play a crucial role in health promotion and chronic disease prevention. Every 5 years, the U.S. Department of Health and Human Services (HHS) and the U.S. Department of Agriculture (USDA) publish the Dietary Guidelines for Americans, the nation's go-to source for nutrition advice. CFR - Code of Federal Regulations Title 21 - Food and Drug ... (a) General requirements. A claim about the calorie or sugar content of a food may only be made on the label or in the labeling of a food if: (1) The claim uses one of the terms defined in this section in accordance with the definition for that term; Constituent Update - July 30, 2021 | Food Safety and ... All ingredients in meat and poultry products must be listed on the label in the ingredients statement, ensuring all allergens will be listed on the product label. The 2004 Food Allergen Labeling and Consumer Protection Act (FALCPA) requires that products under the jurisdiction of the Food and Drug Administration (FDA) that contain a major food ... CFR - Code of Federal Regulations Title 21 - Food and Drug ... (iii) As required in § 101.13 (e) (2) if the food meets these conditions without the benefit of special processing, alteration, formulation, or reformulation to lower the sodium content, it is...
Labeling Guideline on Statements that Bioengineered or ... This guidance document also provides information on how firms can make label or labeling claims that a product was produced from livestock or poultry that were not fed bioengineered or GM feed. This guidance relates to 9 CFR 317.8, 381.129, 412.1(e), 412.1(c)(3), and 412.2. WHMIS 2015 - Labels : OSH Answers Labels will require the following: the pictogram, signal word, and hazard statement are to be grouped together, to be clearly and prominently displayed on the container, to be easy to read (e.g., you can see it easily without using any item except corrective glasses), and to be in contrast with other information on the product or container. USDA Opens Comment Period for Labeling Requirements for ... On September 3, 2021, the U.S. Department of Agriculture, Food Safety and Inspection Service (USDA-FSIS) opened a comment period in advance of a proposed rule on labeling requirements for meat and... Top Reasons Why Having Food Labels is Important | Sticky Biz Offering helpful information on your food labels gives consumers confidence in using your product, especially when it comes to cooking instructions as this gets the best flavor from the food and helps them avoid sickness. We have product specialists ready to help you at 562-945-3486 Categories Custom Labels Tags food labels
CFR - Code of Federal Regulations Title 21 - Food and Drug ... (g) A flavor shall be labeled in the following way when shipped to a food manufacturer or processor (but not a consumer) for use in the manufacture of a fabricated food, unless it is a flavor for...
FDA Food Product Labeling & Packaging Requirements | ESHA ... The Food Allergen Labeling and Consumer Protection Act of 2004 (FALCPA) mandates that packaged food items must declare, in plain language, the presence of any major food allergens (Milk, Egg, Fish, Crustacean shellfish, Tree nuts, Wheat, Peanuts, Soybeans, Sesame) on the product packaging.
Food Allergens - International Regulatory Chart | FARRP ... Key: 1 On April 23, 2021, sesame was added to the priority allergen labeling list (FALCPA) in the U.S. through the passing of the FASTER Act. A compliance date of January 1, 2023 has been set by Congress to allow companies to update allergen control programs and labeling requirements accordingly.
CFR - Code of Federal Regulations Title 21 - Food and Drug ... CFR - Code of Federal Regulations Title 21. The information on this page is current as of Mar 29, 2022. For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). REQUIREMENTS FOR FOREIGN AND DOMESTIC ESTABLISHMENT REGISTRATION AND LISTING FOR HUMAN DRUGS, INCLUDING DRUGS THAT ARE REGULATED UNDER A ...
Expiration Dating And National Drug Code Rules ... In 1972, the United States Congress passed the Drug Listing Act to allow for the Food and Drug Administration (FDA) to have an up-to-date list of all drugs that are commercially distributed. The law stated that all commercially available drugs were to have a unique National Drug Code (NDC).
The USDA's new labeling for genetically modified foods ... Starting Jan. 1, labels at the grocery store are about to get a makeover on foods that have been genetically modified. The goal was to get rid of the patchwork of different labels for foods and...
Introduction to allergen labelling changes (PPDS) | Food ... From 1 October 2021, the requirements for prepacked for direct sale (PPDS) food labelling changed in Wales, England, and Northern Ireland. This labelling helps protect your consumers by providing potentially life-saving allergen information on the packaging. Any business that produces PPDS food is required to label it with the name of the food ...
How To Read Food and Beverage Labels | National Institute ... There are three types of product dates commonly printed on packaged foods and beverages: "Sell by" tells how long the manufacturer suggests that a store should sell items such as meat, poultry, eggs, or milk products. Make sure you buy by this date. "Use by" tells how long items will be at peak quality.
Back to Basics: All About MyPlate Food Groups - USDA Do you remember learning about the food groups in school? You may have been taught using the Food Wheel, Food Guide Pyramid or MyPyramid depending on your age. Kids today learn about the food groups from MyPlate. Now that the back-to-school season is settling down, the nutritionists at MyPlate are offering a back-to-basics refresher lesson on the food groups.
What are the Labeling Requirements for an Artificially ... The regulation on labeling flavors in food is administered by the U.S. Food and Drug Administration (FDA) and can be found in Title 21 of the Code of Federal Regulations (C.F.R.) Section 101.22. The regulation specifies that if a product's label makes a prominent representation with respect to a primary recognizable flavor, then that flavor is deemed to be a "characterizing flavor" and must be ...
What's New with the Nutrition Facts Label | FDA The U.S. Food and Drug Administration (FDA) has updated the Nutrition Facts label on packaged foods and drinks. FDA is requiring changes to the Nutrition Facts label based on updated scientific...
CFR - Code of Federal Regulations Title 21 - Food and Drug ... The requirements in this section apply only to prescription drug products described in § 201.56(b)(1) and must be implemented according to the schedule specified in § 201.56(c), except for the requirement in paragraph (c)(18) of this section to reprint any FDA-approved patient labeling at the end of prescription drug labeling or accompany the ...
Organic on Food Labels - FDA Food products that are ordinarily under FDA's jurisdiction and labeled with organic claims must comply with both USDA NOP regulations for the organic claim and FDA regulations for food labeling and...








Post a Comment for "41 when were food labels required"