42 fda approved drug labels
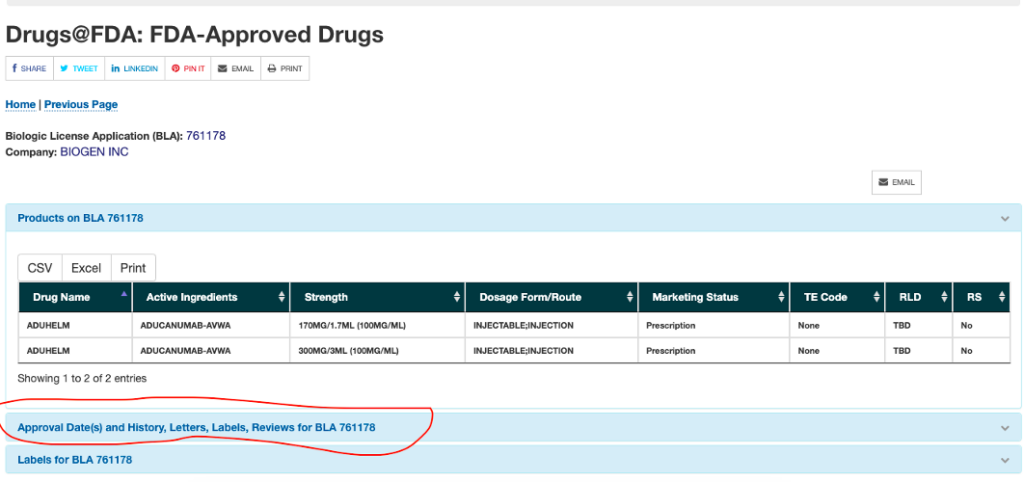
Types of FDA Drug Labeling and Their Requirements - PDG FDA's Guidance for Industry entitled "Help-Seeking" and Other Disease Awareness Communications by or on Behalf of Drug and Device Firms (January 2004) describes two types of drug labeling: FDA-approved labeling, and promotional labeling. [3] An example of FDA-approved labeling is the Professional Package Insert (PPI). FDALabel - U.S. Food and Drug Administration Labeling, Product and Ingredient Identifiers. Application Number for ANDA, BLA, or NDA: 3 to 6 digits (e.g., 077844, 125118, 020977) Unique Ingredient Identifier (UNII): To search for active...
New FDA Drug Approvals for 2022 - Drugs.com Date of Approval: September 27, 2022. Treatment for: Colorectal Cancer, Non-Small Cell Lung Cancer, Glioblastoma Multiforme, Renal Cell Carcinoma, Cervical Cancer, Ovarian Cancer, Fallopian Tube Cancer, Peritoneal Cancer. Vegzelma (bevacizumab-adcd) is a vascular endothelial growth factor (VEGF) inhibitor biosimilar to Avastin (bevacizumab) for ...

Fda approved drug labels
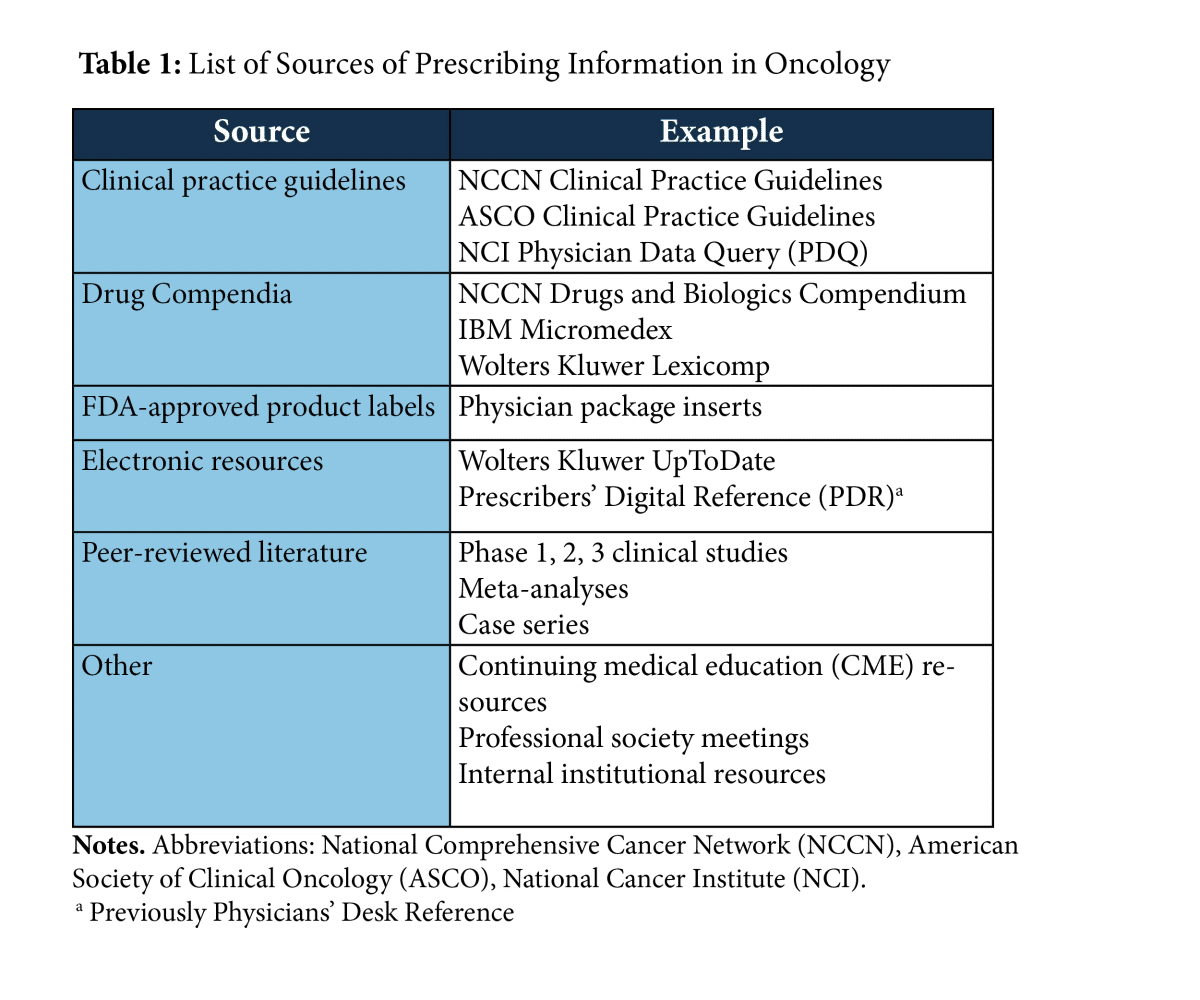
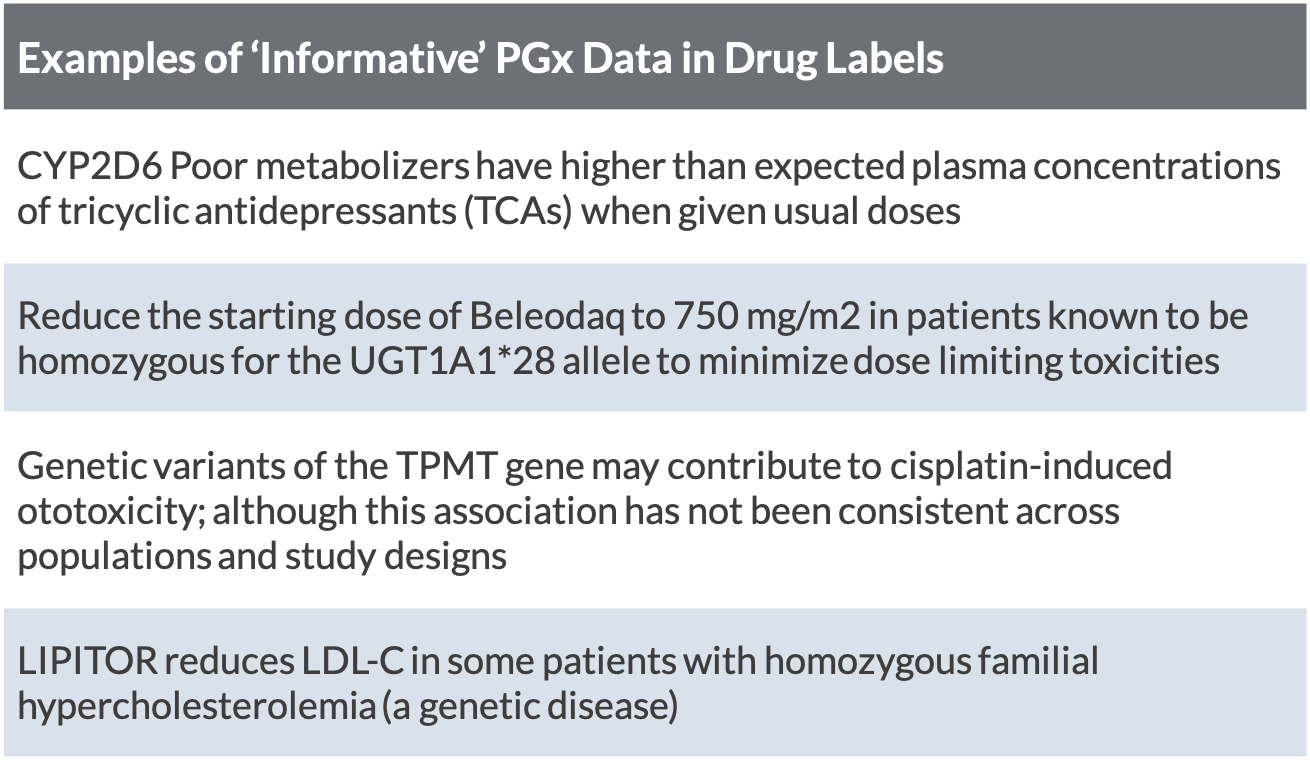
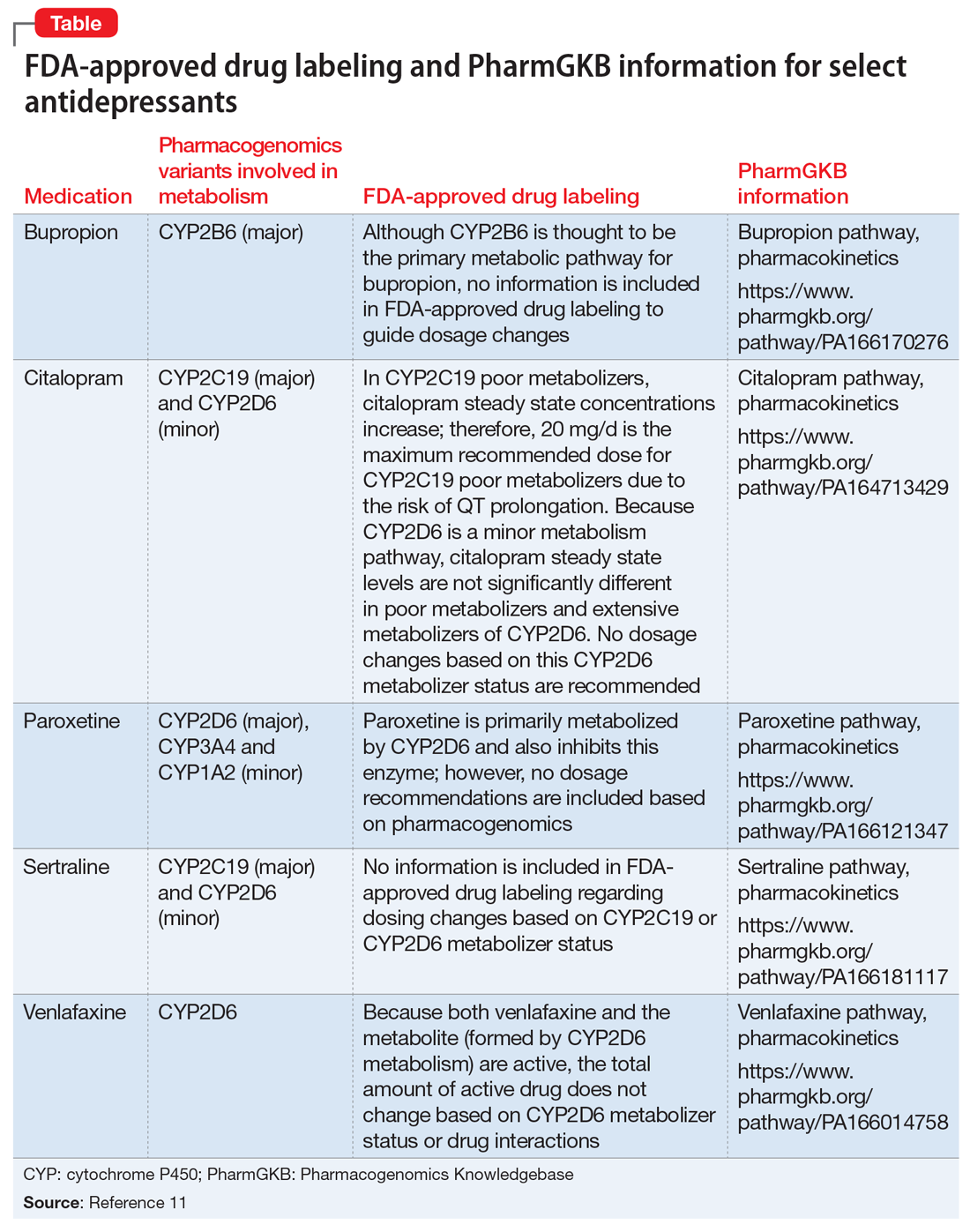
Table of Pharmacogenomic Biomarkers in Drug Labeling | FDA Drug labeling may contain information on genomic biomarkers and can describe: The table below ... FDA Label Search - Food and Drug Administration The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) The drug labeling and other information has been reformatted to make it easier to read but its content has neither been altered nor ... PDF HIGHLIGHTS OF PRESCRIBING INFORMATION The recommended dose is 2.5 mg ... Discontinue drug or discontinue nursing. (8.2) Severe Hepatic Impairment: Not recommended. (8.7, 12.2) See 17 for PATIENT COUNSELING INFORMATION and Medication Guide. Revised: 04/2021 . FULL PRESCRIBING INFORMATION: CONTENTS* WARNING: (A) PREMATURE DISCONTINUATION OF ELIQUIS. INCREASES THE RISK OF THROMBOTIC EVENTS (B) SPINAL/EPIDURAL HEMATOMA
Fda approved drug labels. PDF ADDERALL (CII) - Food and Drug Administration In addition, drugs that effect urinary pH are known to . NDA 11-522/S-040 Page 5 alter the elimination of amphetamine, and any decrease in amphetamine's metabolism that might occur due to drug interactions or genetic polymorphisms is more likely to be clinically significant when renal PDF Reference ID: 3397413 - Food and Drug Administration antipsychotic drugs are at an increased risk of death. SEROQUEL is not approved for elderly patients with dementia-related psychosis (5.1) Suicidal Thoughts and Behaviors • Increased risk of suicidal thoughts and behavior in children, adolescents and young adults taking antidepressants (5.2) • FDALabel: Full-Text Search of Drug Product Labeling | FDA FDALabel Database is a web-based application that allows users to perform customizable searches of a ... PDF Lipitor (atorvastatin calcium) Label - Food and Drug Administration FDA-approved patient labeling. Revised: 4/2019 : Reference ID: 4418807 _____ FULL PRESCRIBING INFORMATION: CONTENTS* 1 INDICATIONS AND USAGE : 1.1 Prevention of Cardiovascular Disease in Adults 1.2 Hyperlipidemia ... Drug therapy is recommended as an adjunct to diet
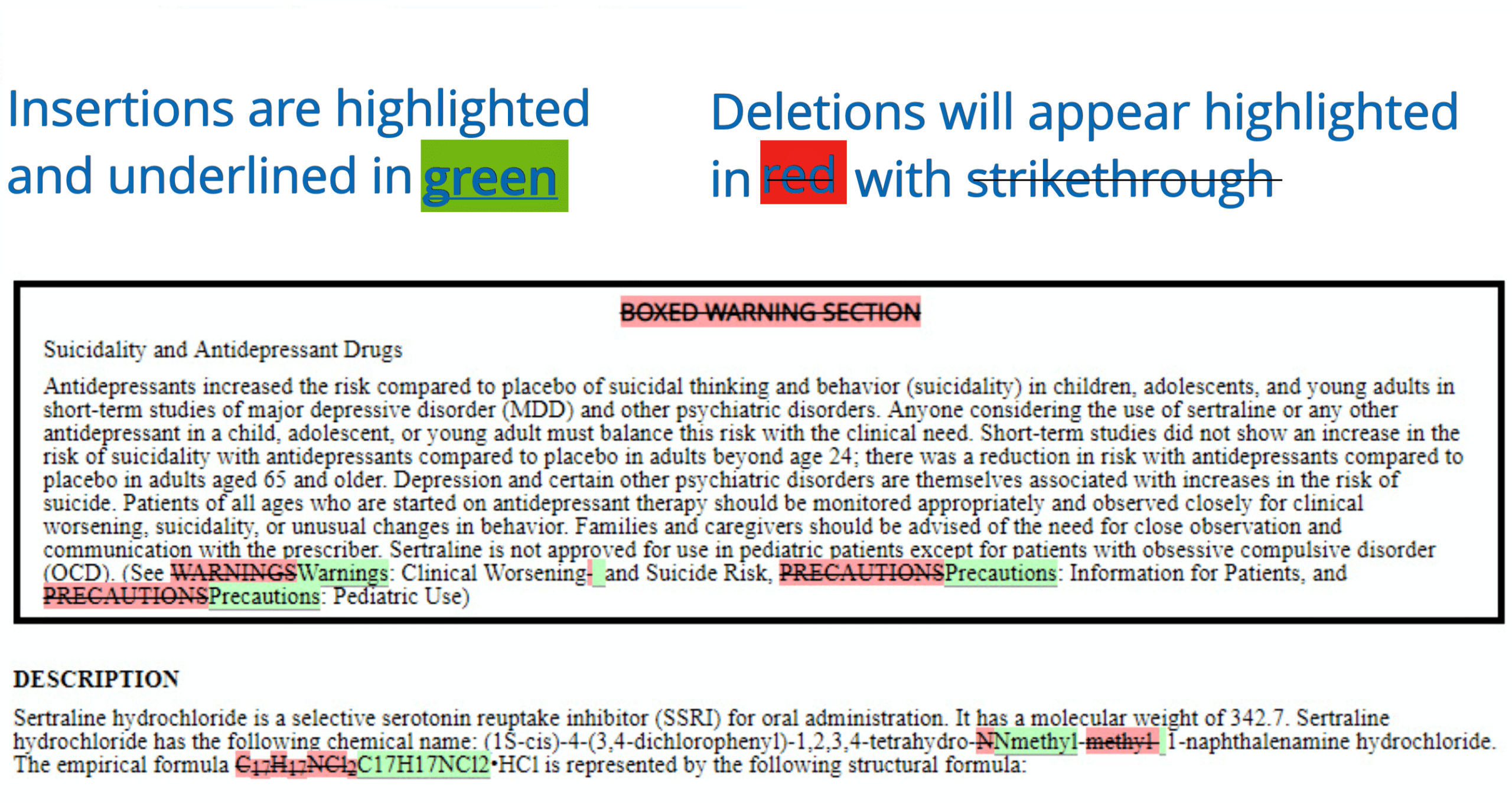
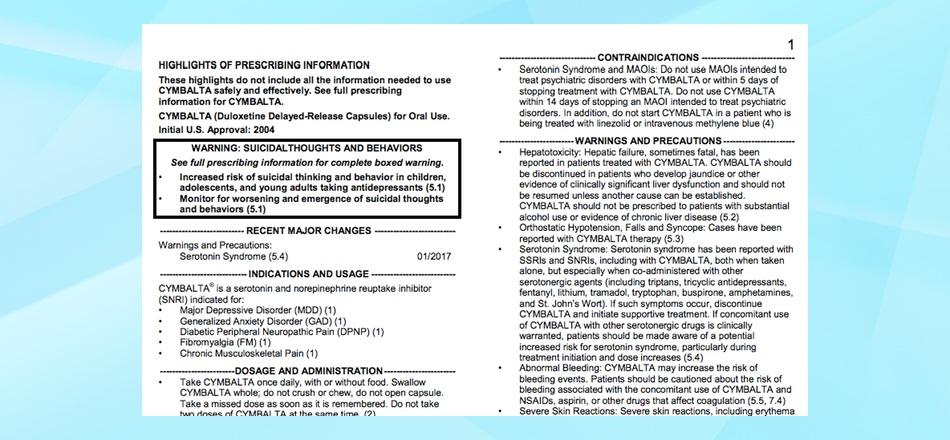
Drug Approval and Labeling | Cancer.Net Off-label use. When the FDA approves a new drug, it only approves the drug to treat a specific condition when given in the manner described on the drug's label. Drug makers may still do more research on other uses for the drug, such as treating another type of cancer. A doctor may prescribe an FDA-approved drug to treat a condition not listed ... PDF Depakote (divalproex sodium) Tablets - Food and Drug Administration FDA Approved Labeling Text dated October 7, 2011 Page 2 of 57 FULL PRESCRIBING INFORMATION: CONTENTS* BOXED WARNING 1 INDICATIONS AND USAGE 1.1 Mania 1.2 Epilepsy 1.3 Migraine 2 DOSAGE AND ADMINISTRATION 2.1 Mania 2.2 Epilepsy 2.3 Migraine 2.4 General Dosing Advice 3 DOSAGE FORMS AND STRENGTHS 4 CONTRAINDICATIONS 5 WARNINGS AND PRECAUTIONS FDA's Labeling Resources for Human Prescription Drugs | FDA Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective use of the drug; and (2) includes the Prescribing Information,... Drug Labeling Overview - Food and Drug Administration The openFDA drug product labeling API returns data from this dataset. The labeling is a 'living document' that changes over time to reflect increased knowledge about the safety and effectiveness of...
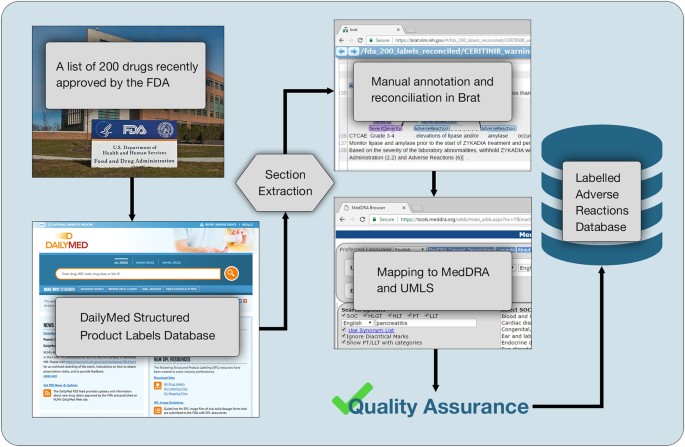
Alnylam Announces FDA Approval of Supplemental New Drug Application for ... The supplemental New Drug Application also included results from the open-label extensions of the ILLUMINATE-A and ILLUMINATE-B Phase 3 studies of pediatric and adult patients with PH1. The label has correspondingly been updated to highlight the maintenance of sustained reductions in UOx through Month 24 and Month 12, respectively. Drug Labels | FDA Drug Labels This is a partial collection of labeling submitted to the FDA Center for Veterinary Medicine (FDA CVM) by animal drug sponsors for Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and... About Oncology Prescription Drug Labeling | FDA Maintaining FDA-approved USPI occurs throughout the drug's lifecycle. After initial approval, companies are required to update their USPI when new information becomes available that causes the USPI... Pharmacogenomic Biomarkers in US FDA-Approved Drug Labels ... - PubMed Pharmacogenomic Biomarkers in US FDA-Approved Drug Labels (2000-2020) Pharmacogenomics (PGx) is a key subset of precision medicine that relates genomic variation to individual response to pharmacotherapy. We assessed longitudinal trends in US FDA approval of new drugs labeled with PGx information.
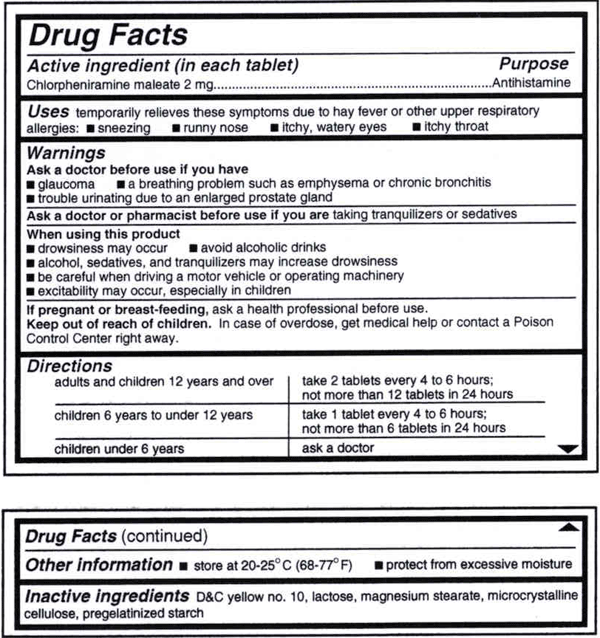
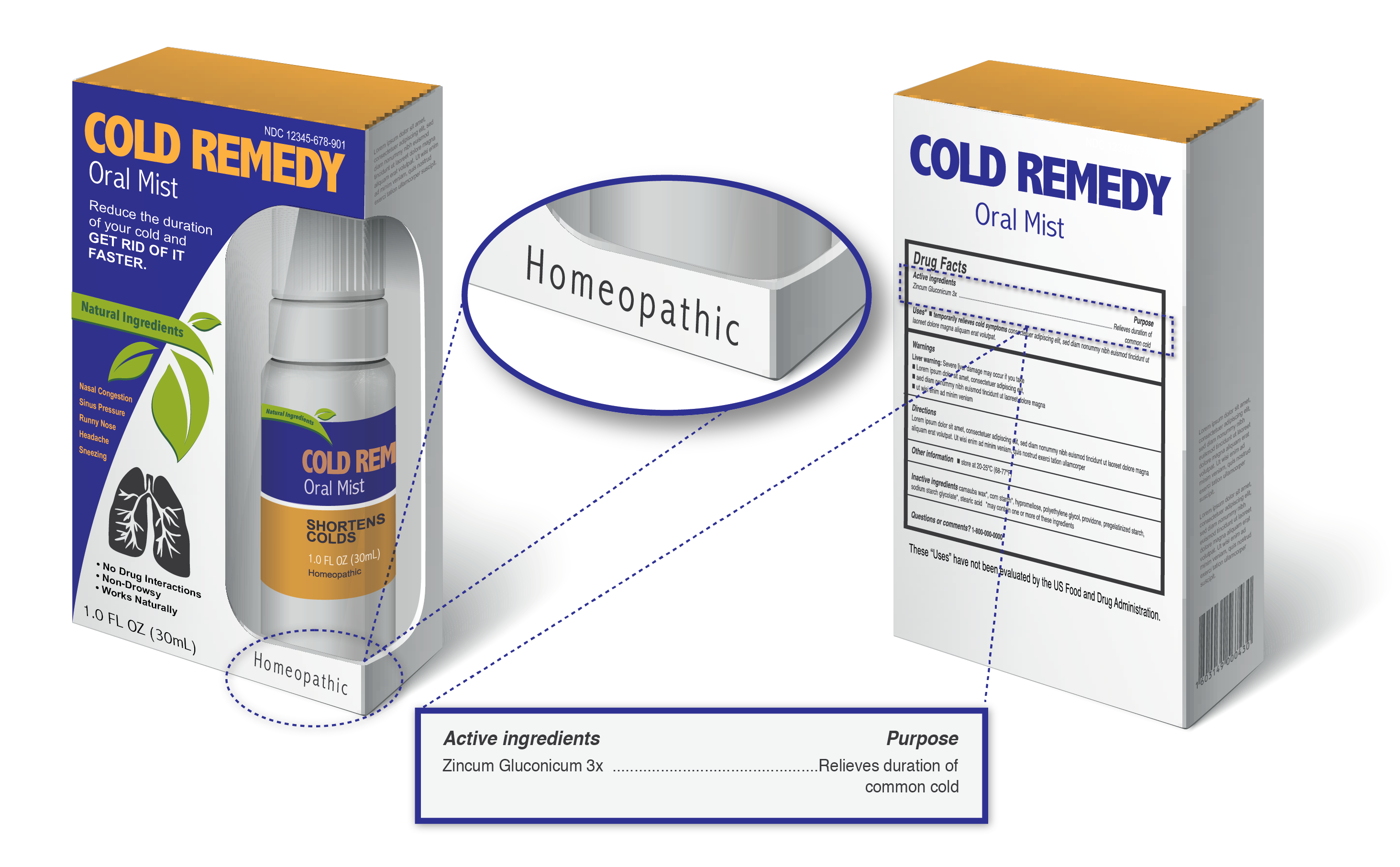
OTC Drug Facts Label | FDA In the Federal Register of March 1999, the Food and Drug Administration published the OTC Drug Facts Label regulation. This regulation required most OTC drug products to comply with the new format...
What Information Should Be on Drug Labels? - MedicineNet The FDA requires prescription labeling to be printed with: Pharmacy information The doctor's information Instructions Physical description of the drug Federal caution statement Dates Pharmacy prescription number Number of pills Number of times the drug can be reordered
DailyMed The National Library of Medicine (NLM)'s DailyMed searchable database provides the most recent labeling submitted to the Food and Drug Administration (FDA) by companies and currently in use (i.e., "in use" labeling).
PDF TOPAMAX (topiramate) Label - Food and Drug Administration Suicidal behavior and ideation: antiepileptic drugs increase the risk of suicidal behavior or ideation (5.5) Cognitive/neuropsychiatric adverse reactions: use caution when operating machinery including cars; depression and mood problems may occur (5.6) Fetal Toxicity: use during pregnancy can cause cleft lip and/or palate and
PDF Neurontin (gabapentin) Capsules Neurontin (gabapentin) Tablets ... FDA Approved Labeling Text dated 03/01/2011 Page 3 . Elimination: Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing.
DailyMed - FDA Resources: SPL, Other Prescription Drug Labeling ... FDA's Prescription Drug Labeling Resources. This website provides over 100 labeling resources for the Prescribing Information, FDA-approved patient labeling, and/or carton and container labeling for human prescription drugs, including biological products. FDA's Drug Guidances. Guidance documents represent the FDA's current thinking on a ...
Drug Safety-related Labeling Changes (SrLC) Database Labeling for generic drugs regulated under ANDAs. Labeling for FDA-approved prescription products regulated by the Center for Biologics Evaluation and Research (for example, vaccines, allergenic...
Labeling Information | Drug Products | FDA For prescription drug labeling resources (e.g., Prescribing Information, FDA-approved patient labeling, and carton and container labeling), please see the Prescription Drug Labeling Resources web ...
PDF HIGHLIGHTS OF PRESCRIBING INFORMATION The recommended dose is 2.5 mg ... Discontinue drug or discontinue nursing. (8.2) Severe Hepatic Impairment: Not recommended. (8.7, 12.2) See 17 for PATIENT COUNSELING INFORMATION and Medication Guide. Revised: 04/2021 . FULL PRESCRIBING INFORMATION: CONTENTS* WARNING: (A) PREMATURE DISCONTINUATION OF ELIQUIS. INCREASES THE RISK OF THROMBOTIC EVENTS (B) SPINAL/EPIDURAL HEMATOMA
FDA Label Search - Food and Drug Administration The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) The drug labeling and other information has been reformatted to make it easier to read but its content has neither been altered nor ...
Table of Pharmacogenomic Biomarkers in Drug Labeling | FDA Drug labeling may contain information on genomic biomarkers and can describe: The table below ...
:no_upscale()/cdn.vox-cdn.com/uploads/chorus_asset/file/4097058/flibanserin%20with%20DH%20v4%20(post-label)%20(1).jpg)
.jpg)







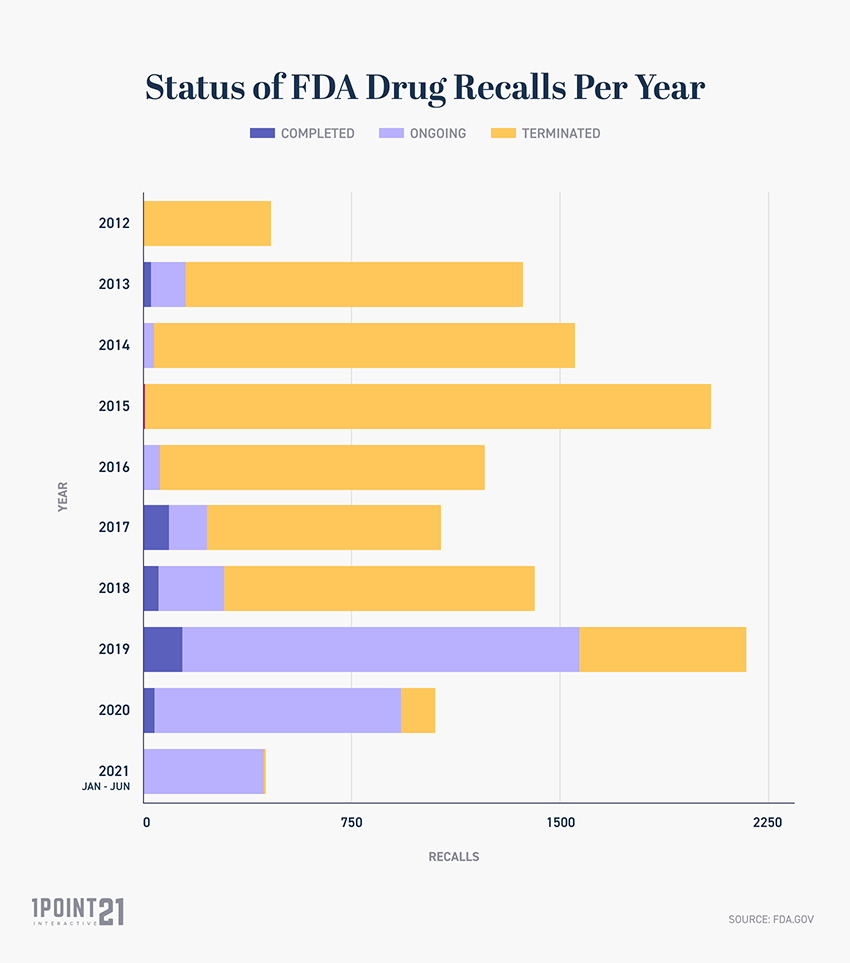


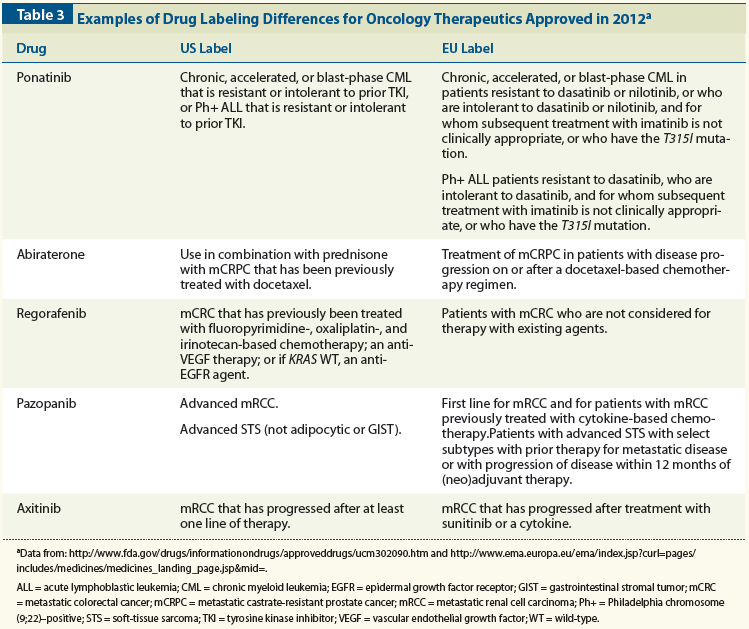












Post a Comment for "42 fda approved drug labels"